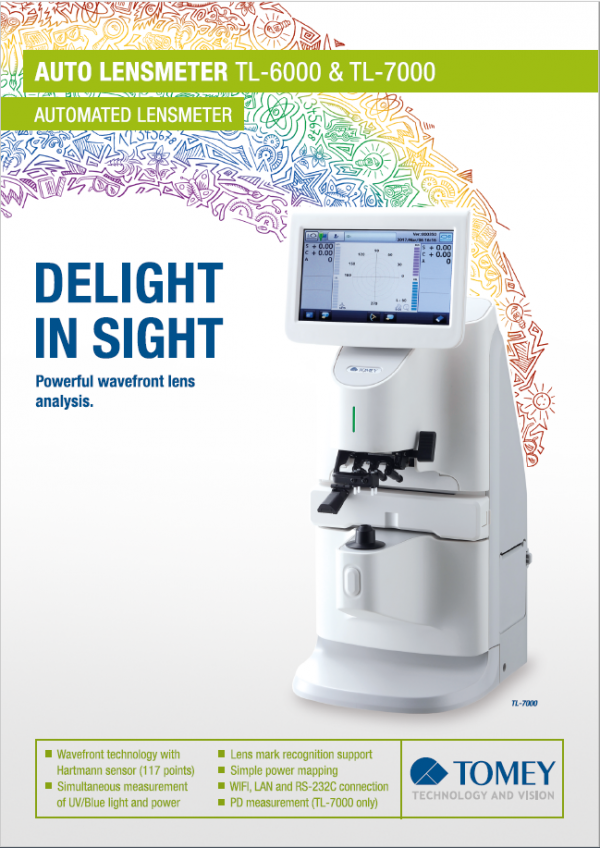
| Compliant with GMP
Productivity – Provides continuous high capacity decontamination of large spaces, >20,000ft3, such as clean rooms, production filling line isolators, pass-through chambers, etc.
Ease of Use – Can be integrated with building automation systems.
Flexibility – Can be conveniently integrated into pass-through chamber or aseptic filling systems or used to decontaminate entire suites or rooms in sequence.
Efficacy – The gaseous decontamination process is effective against a wide variety of microorganisms and has a broad range of material compatibility.
Green – Provides a non-toxic and environmentally friendly alternative to other decontamination processes. Vaporized Hydrogen Peroxide breaks down to water vapor and Oxygen.
Secure – Write text saying Optional 21 CFR Part 11 data security package enables audit trail electronic data capture, user administration, and other features for use and validation.
Connectivity – Optional .. |